Evidence summary and stakeholder input
The following is a summary of the findings from the evidence gathering activities and stakeholder consultations undertaken to inform development of the Roadmap. For more information on the development of the Roadmap see the About page.
Last updated: 31 March 2022
Research
Evidence summary
Research that spans the continuum of pancreatic cancer prevention, treatment, and care is essential to drive improvements in pancreatic cancer outcomes.
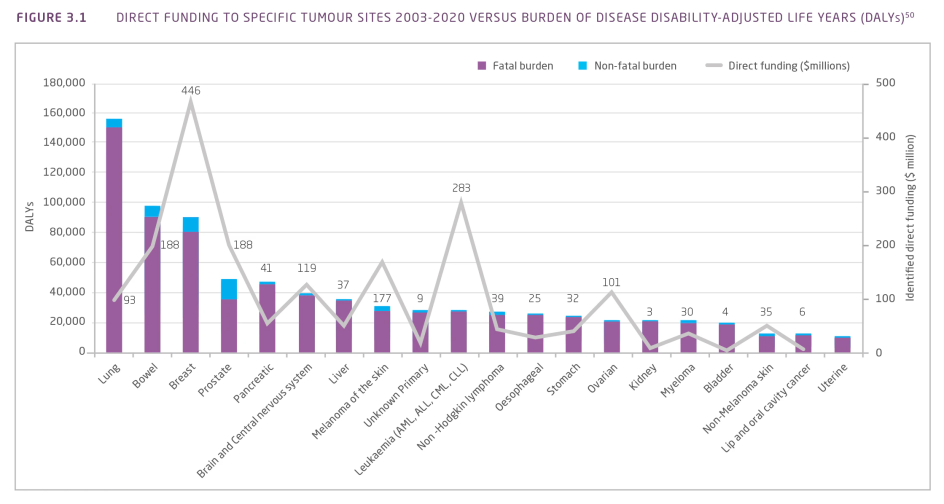
During the period 2003-2005 to 2018-2020 $40 million was provided to 145 pancreatic cancer research projects and research programs in Australia. While this represented a 20-fold increase in funding and more than a 9-fold increase in the number of research projects over this timeframe, funding to pancreatic cancer research was proportionally low compared with its burden on the Australian population (Figure 3.1).
The Australian Government provided more than half (56%; $22.3m) of all pancreatic cancer research funding and 90% of pancreatic cancer research projects were funded by a single funding source.
Figure 3.1 Direct funding to specific tumour sites 2003-2020 versus burden of disease (DALYs)¹
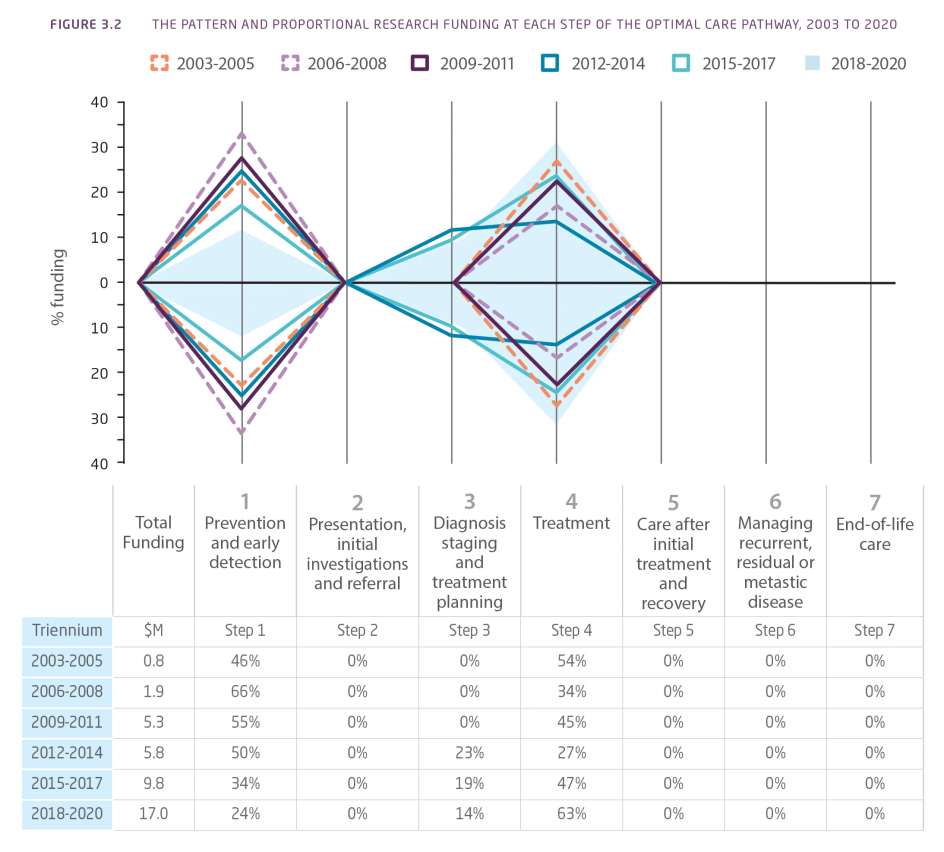
In the period 2003-2020, no research investment was identified in the areas of presentation, initial investigations and referral, care after initial treatment and recovery, managing recurrent, residual, or metastatic disease, or end-of-life care (Figure 3.2). While there were proportional changes in funding, the total amount of funding for pancreatic cancer research in Australia increased in the areas of prevention and early detection, diagnosis, staging and treatment planning, and treatment.
More than half (53%; 81) of all research projects involved one or more named co-investigators, but only 9% of research projects had a named international co-investigator.
Figure 3.2 The pattern and proportional research funding at each step of the Optimal Care Pathway (OCP), 2003 to 2020
Stakeholder input
Stakeholders highlighted the need for increased, focused and continued funding for pancreatic research and clinical trials and the importance of having a balanced portfolio of research funding across the continuum. The collaboration of funders and exploring international funding opportunities were strongly supported.
To realise significant improvements in survival, stakeholders highlighted the importance of research to understand disease aetiology, to identify better methods for early detection and treatment, and to understand mechanisms of treatment response, resistance and disease recurrence. Survivorship and palliative care research together with research into the needs of families, carers and priority population groups was highlighted. Health-services research and translational/implementation science to optimise survival and quality-of-life outcomes were also raised.
The importance of a research focus on understanding the link between pancreatic cancer and diabetes, screening, early detection, and surveillance, the prevalence of recurrent, residual and metastatic disease and its management were also raised by Aboriginal and Torres Strait Islander stakeholders. The inclusion and participation of people from culturally, ethnically and linguistically diverse backgrounds in primary health care research, and sharing specific stories and experiences of Aboriginal and Torres Strait Islander people with pancreatic cancer were highlighted.
Increased public awareness of the importance of tissue banking and biopsies for research, funding for tissue sampling, expansion of available infrastructure, and mechanisms to support access and use of technologies such as biobanks, organoids and patient-derived xenografts were raised by stakeholders.
Pancreatic stakeholders noted that existing national and international networks can be leveraged and built upon to address critical questions and areas of unmet need in pancreatic cancer, and a strengthened coordination of the research effort, including collaboration across research groups, government, industry and philanthropic sectors, was strongly supported. Facilitating national forums with scientific and clinical researchers to share progress and accelerate idea generation was also raised.
A key priority for pancreatic stakeholders is the targeted and sustained funding for a strategic national program of pancreatic cancer research that fosters a collaborative program of preclinical and clinical research to drive improvements in outcomes, facilitates meaningful consumer engagement to ensure that research is responsive to the needs of patients and carers, incorporates industry engagement, leverages technology, and requires and rewards national and international collaboration.
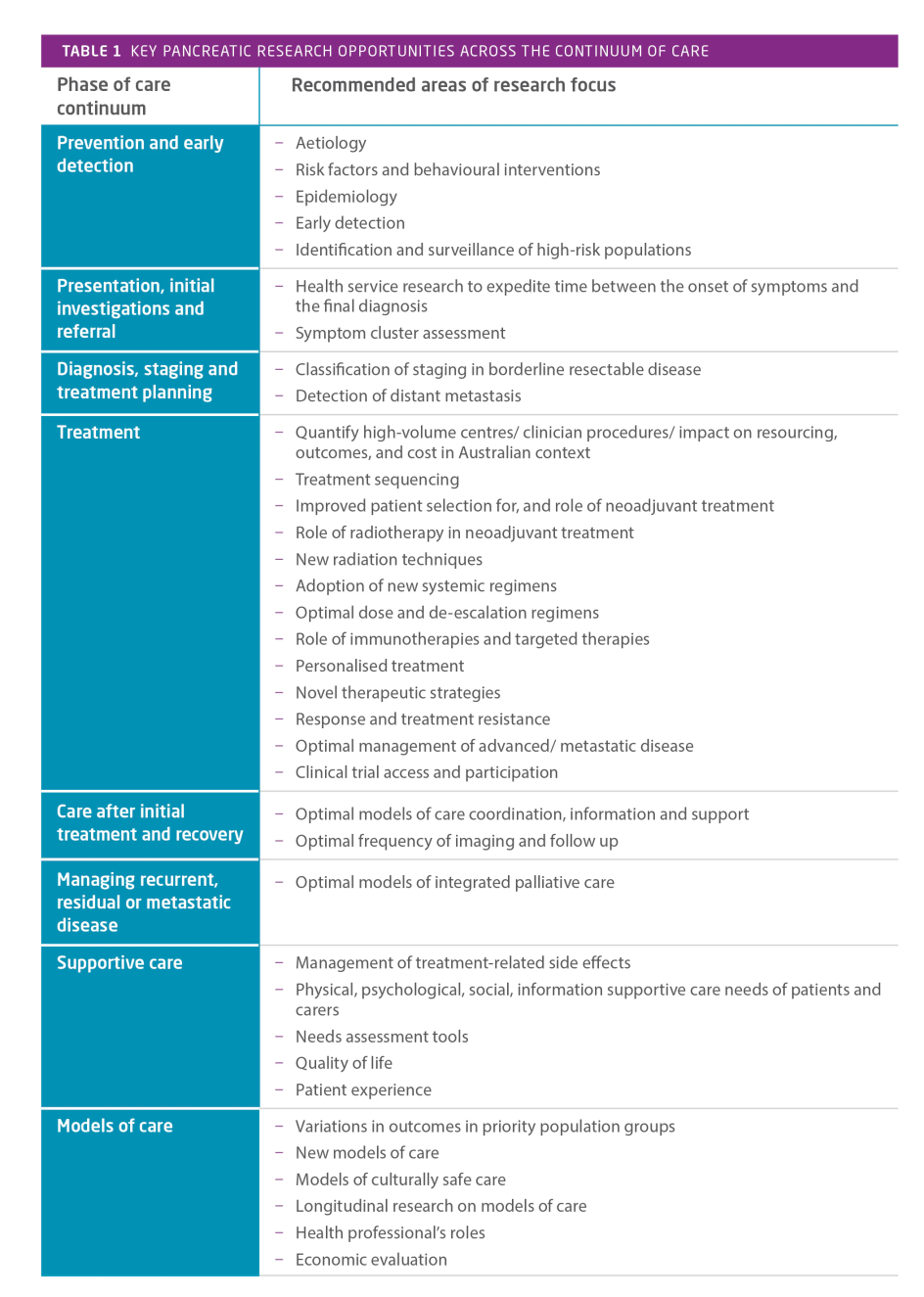
The evidence gathering and stakeholder engagement activities surfaced a substantial number of research opportunities across all stages of the continuum of care. The significant emphasis on research underscores the paucity of existing evidence and critical need for research to drive improvements in pancreatic cancer outcomes. The key areas of research focus across the continuum of care are summarised in Table 1. and for each area a number of specific research opportunities were raised.
Clinical trials
Evidence summary
Clinical trials benefit the community by improving the survival of people affected by cancer and contribute to a reduction in premature death and disability. Clinical trials help generate evidence for best-practice cancer care and are fundamental to establishing whether new cancer treatments, diagnostic tests or preventive interventions are effective.
The pancreatic cancer optimal care pathway (OCP) recommends that all patients be given the opportunity to participate in relevant clinical trials where available. Data from the Upper Gastrointestinal Cancer Registry (UGICR) indicates only 8% of patients with pancreatic cancer participated in a clinical trial and less than 1% of patients aged over 85 were involved in a clinical trial. A higher proportion of patients participated in a clinical trial if they had been presented to a multidisciplinary meeting (MDM; 11% vs. 4% not presented). Those who did participate in clinical trials tended to be younger, from higher socioeconomic advantage, born in Australia, located in major cities and had non-metastatic disease.
Between 2012 and 2020 in Australia, 148 pancreatic clinical trials were identified, 60 of which focused exclusively on pancreatic cancer. Of these, 31 were investigator-driven and 29 were industry-driven trials.
The majority of investigator-initiated trials were intervention trials, phase 2, conducted in a single, Australian investigational site. These trials focused on diagnosis, staging and treatment planning, and first-line chemotherapy and/or radiotherapy-related interventions. The trials were inclusive of participants with all stages of pancreatic cancer and the main outcomes of interest were survival, intervention feasibility and tumour progression.
In contrast the majority of industry-driven trials while also intervention trials, were phase 3, conducted both in Australia and internationally. These trials focused on treatment for recurrent, residual or metastatic disease and principally included patients with metastatic disease. The main outcomes of interest were survival and adverse effects.
A larger proportion of industry trials compared with investigator-initiated (79% vs. 6%) recruited participants from across multiple Australian states, however, the majority of pancreatic cancer trials were conducted in metropolitan Australia alone. Only a small proportion of investigator-initiated and industry trials, 6% vs. 38% respectively, recruited participants from regional areas.
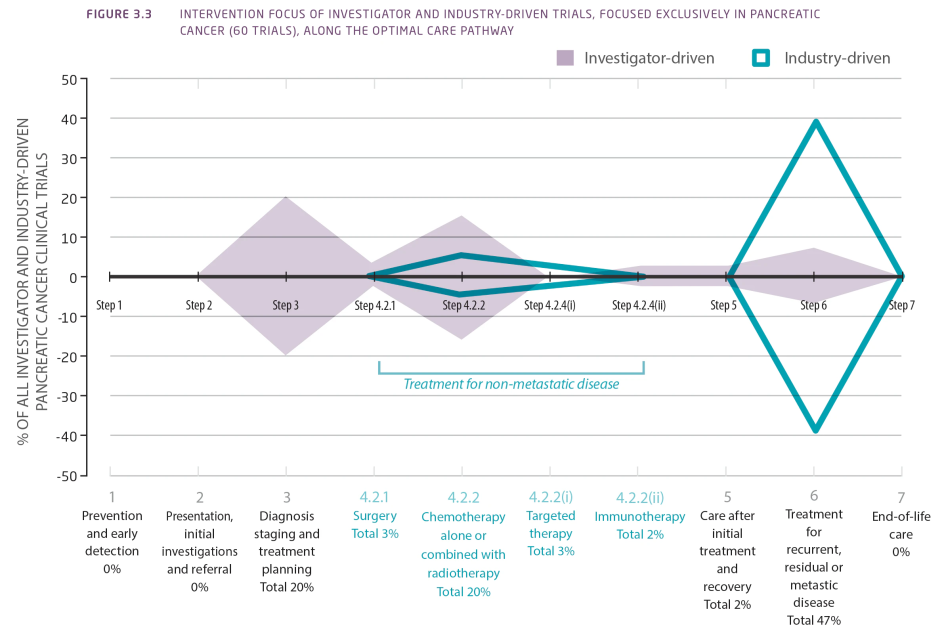
Investigator-driven clinical trials predominantly focused on interventions in the earlier steps of the OCP while industry-driven trials had a greater focus on the treatment of recurrent, residual or metastatic disease (Figure 3.3).
Figure 3.3 Intervention focus of investigator and industry-driven trials, focused exclusively in pancreatic cancer (60 trials), along the optimal care pathway
The measurement of patient-reported outcomes, such as quality of life and symptom burden in the trials assessed was limited, and the active inclusion of priority populations such as Aboriginal and Torres Strait Islander people, culturally and linguistically diverse (CALD) people and people living in lower socioeconomic areas was not specified in the registered inclusion criteria of the identified trials. Consequently, it is difficult to ascertain if the clinical trials included and represented the wider pancreatic cancer population across Australia.
Stakeholder input
Stakeholders raised the importance of increased awareness, promotion and participation in clinical trials, including active promotion, inclusion and participation of people from culturally, ethnically and linguistically diverse backgrounds in pancreatic cancer clinical trials. Barriers to Aboriginal and Torres Strait Islander participation in clinical trials included lack of awareness, understanding of clinical trial information, consent processes, location and number of visits, cultural safety and support.
Stakeholders supported equitable access to clinical trials, improved access to trials in rural and remote areas, and the use of technology and teletrials to improve geographic access.
The development of a standardised approach for streamlined recruitment into clinical trials, the education of health professionals about access to, and the benefits of, clinical trials for pancreatic cancer, national and international collaborative opportunities to conduct larger investigator-driven trials and facilitate higher participation rates, and the requirement for international collaboration and engagement with industry to ensure that Australians can access novel therapeutics and benefit from trial participation were also highlighted.
Key priority areas, strategies and activities
Each key priority area has supporting strategies and associated activities for implementation in the short, medium or long term that have been identified to achieve the priority.
References
1. Australian Institute of Health and Welfare 2019. Australian Burden of Disease Study: impact and causes of illness and death in Australia 2015. Cat. no. BOD 25. Canberra: AIHW.

